Prostate lineage-specific metabolism governs luminal differentiation and response to antiandrogen treatment
In a study published in Nature Cell Biology, first and second authors Jenna Giafaglione and Preston Crowell identified a role for pyruvate/lactate metabolism in the epigenetic regulation of prostate epithelial identity (differentiation). The more “luminal-like” a tumor is, the more likely it is to respond to hormone therapy. This process is regulated at the epigenetic level. We wanted to understand if/how upstream metabolism is involved. We performed the first metabolic profiling and nutrient tracing in distinct prostate epithelial cell-types. We found that basal and luminal cells use glucose differently, especially when it comes to how they generate and metabolize citrate. This is likely related to the fact that luminal cells have a high demand for citrate both for secretion and lipid synthesis. We found that when basal cells differentiate into luminal cells, they upregulate an oxidative phosphorylation signature and increase incorporation of glucose-derived carbons into the TCA cycle, indicative of pyruvate oxidation. Pharmacologic or genetic blockade of pyruvate oxidation (targeting the mitochondrial pyruvate carrier) impairs basal to luminal differentiation and reduces luminal features in prostate cancer. Suppression of pyruvate oxidation promotes the intracellular accumulation of lactate, which impairs luminal differentiation likely through inhibition of histone deacetylation. Blocking pyruvate oxidation or supplementing with lactate alters chromatin accessibility at lineage-specific genes. Clinical prostate tumors that exhibit resistance to androgen receptor inhibitors express low levels of the mitochondrial pyruvate carrier. We found that inhibition of pyruvate oxidation, or lactate supplementation, can reduce sensitivity to androgen receptor inhibitors. Our take-home messages are: (1) Studying normal cells and their differentiation gave us insight into prostate cancer. (2) Metabolism can play an important role in regulating treatment response in prostate cancer, so we should consider how local and systemic factors influence prostate cancer metabolism.
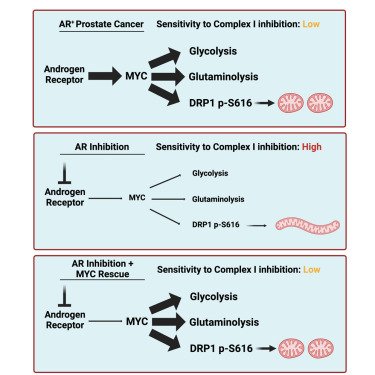
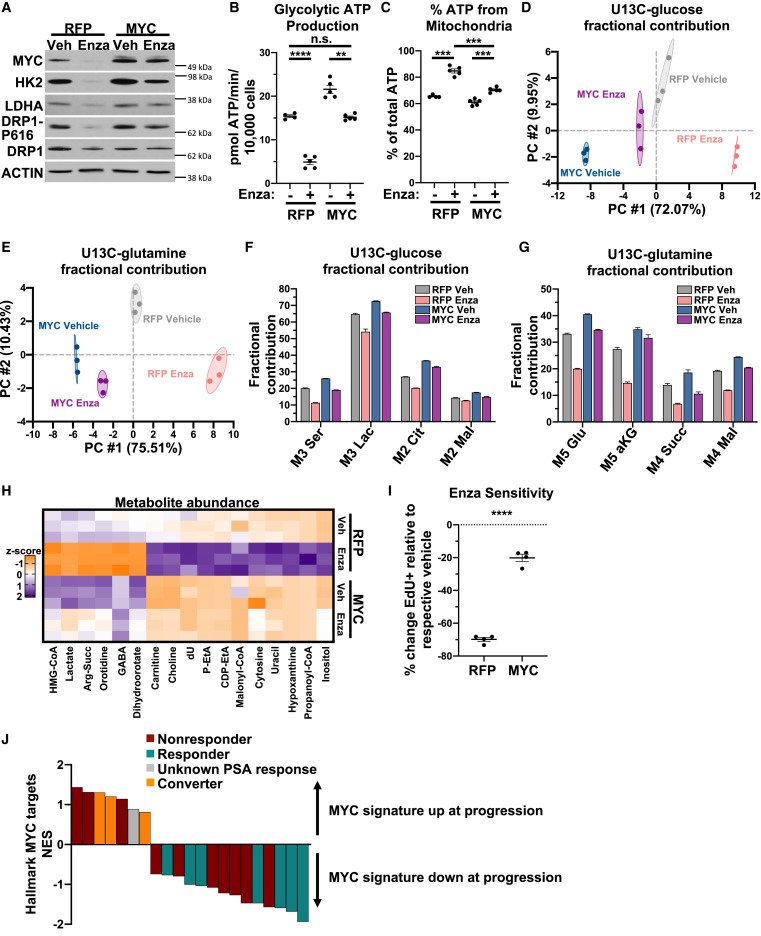
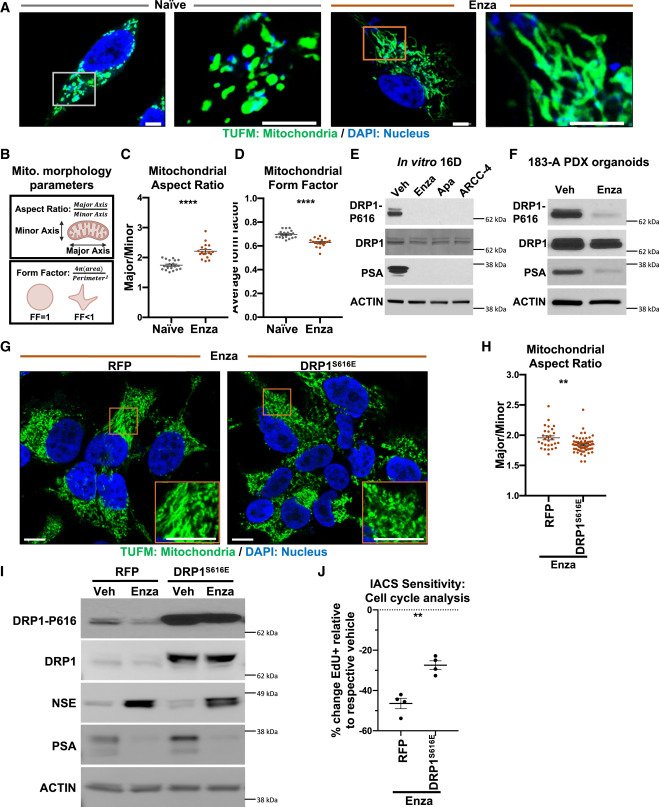
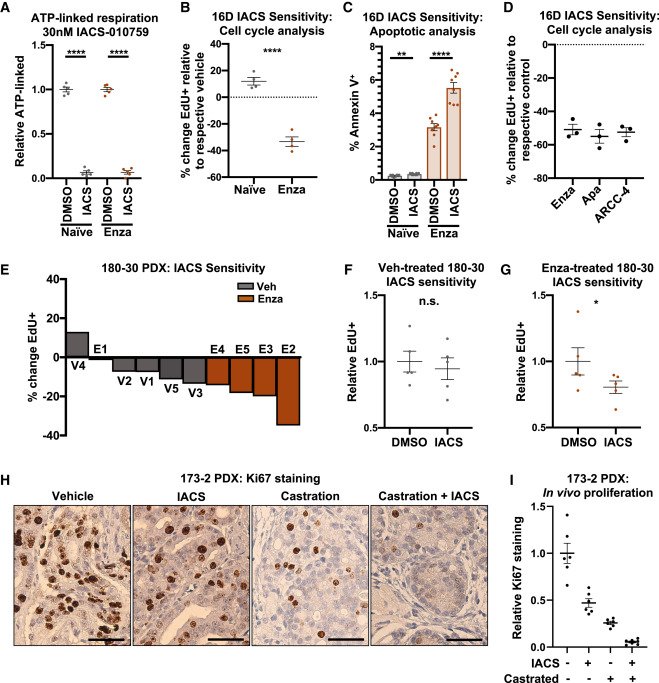
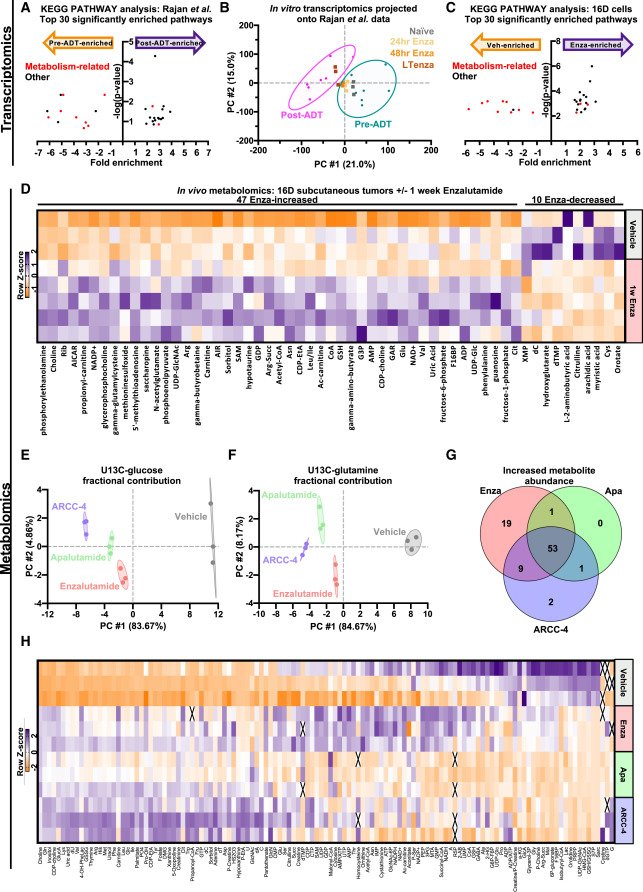
MYC is a regulator of androgen receptor inhibition-induced metabolic requirements in prostate cancer
Advanced prostate cancers are treated with therapies targeting the androgen receptor (AR) signaling pathway. While many tumors initially respond to AR inhibition, nearly all develop resistance. It is critical to understand how prostate tumor cells respond to AR inhibition in order to exploit therapy-induced phenotypes prior to the outgrowth of treatment-resistant disease. In a study published in Cell Reports, we comprehensively characterize the effects of AR blockade on prostate cancer metabolism using transcriptomics, metabolomics, and bioenergetics approaches. The metabolic response to AR inhibition is defined by reduced glycolysis, robust elongation of mitochondria, and increased reliance on mitochondrial oxidative metabolism. We establish DRP1 activity and MYC signaling as mediators of AR-blockade-induced metabolic phenotypes. Rescuing DRP1 phosphorylation after AR inhibition restores mitochondrial fission, while rescuing MYC restores glycolytic activity and prevents sensitivity to complex I inhibition. Our study provides insight into the regulation of treatment-induced metabolic phenotypes and vulnerabilities in prostate cancer.
Andrew Goldstein, associate professor of molecular, cell and developmental biology and urology at the David Geffen School of Medicine at UCLA, received a $900,000 Idea Development Award from the Department of Defense to study how metabolism changes in prostate cancer as a result of hormone therapy, the most commonly used treatment for men with advanced disease.
Hormone therapy, also known as antiandrogen therapy, can help stop prostate cancer cell growth for some time. But treatment alone isn’t a cure. Nearly all patients eventually see their cancer progress, making further improvements vital to improve clinical outcomes.
The award will help Goldstein and his team research ways to target metabolism that occurs in the mitochondria, which play a key role in cells that initially survive hormone therapy. It will also help the researchers better understand how tumors survive and grow even after treatment with drugs that target the androgen receptor — by focusing on how surviving tumor cells generate the energy required to grow.
“While we still have a lot to learn, we hope this work can one day lead to new clinical trials to improve the treatment of prostate cancer and hopefully prevent or delay the progression of the disease and ultimately reduce deaths from the disease,” said Goldstein, a member of the UCLA Jonsson Comprehensive Cancer and the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA.
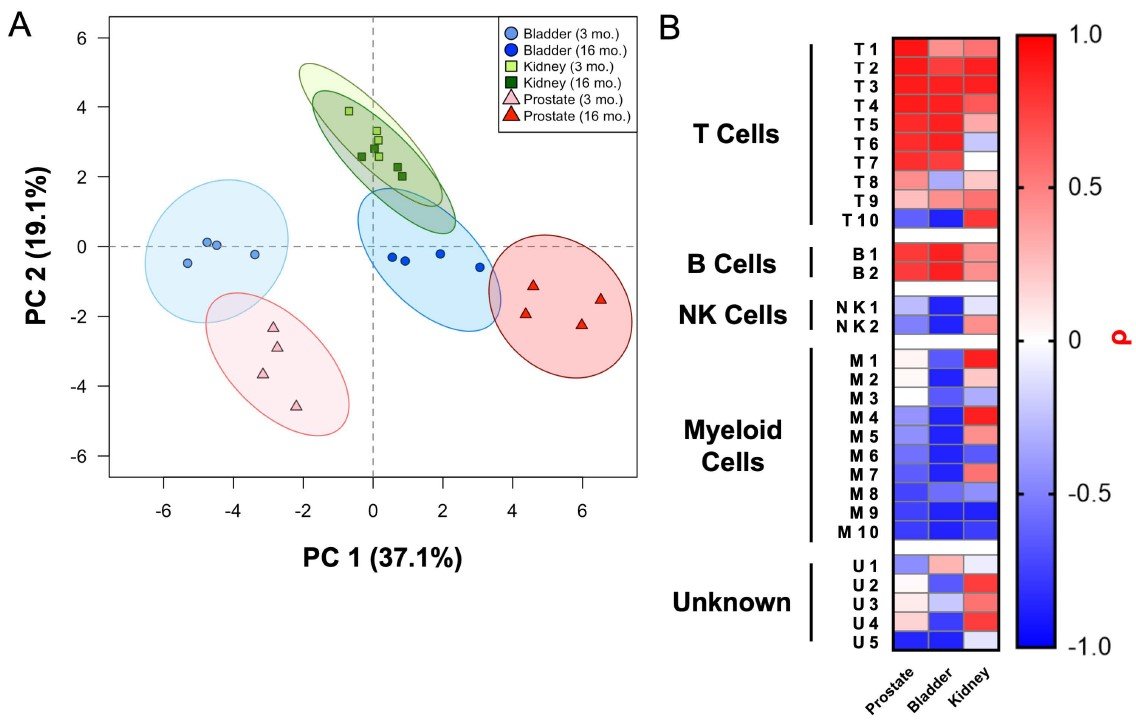
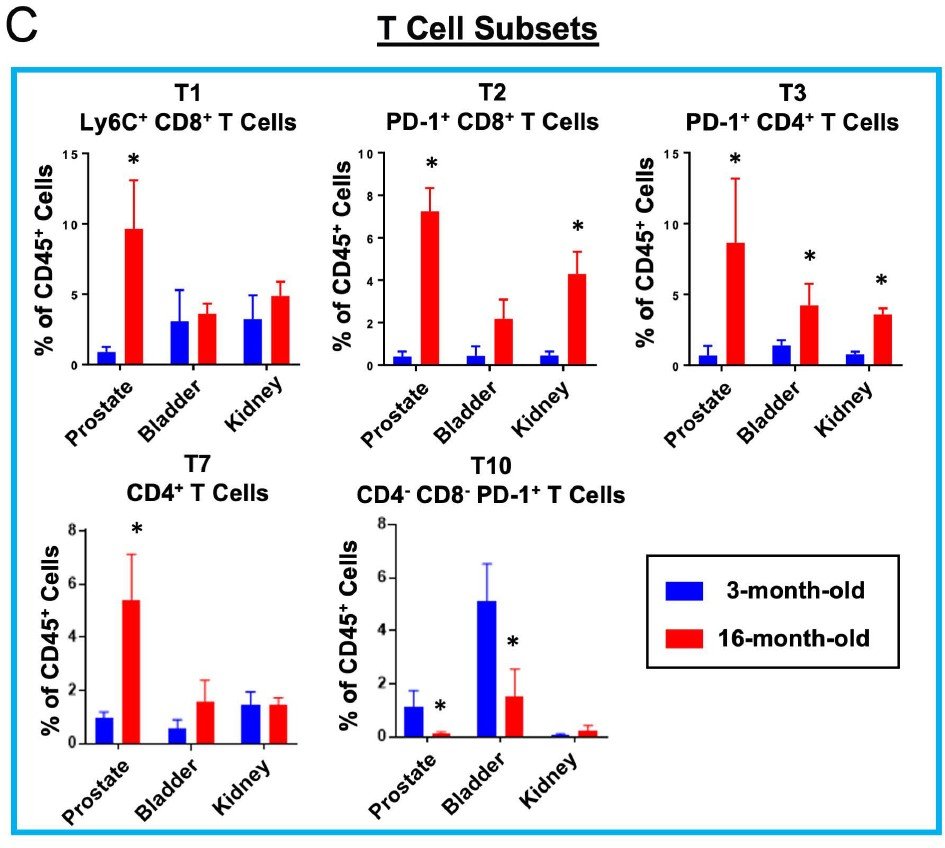
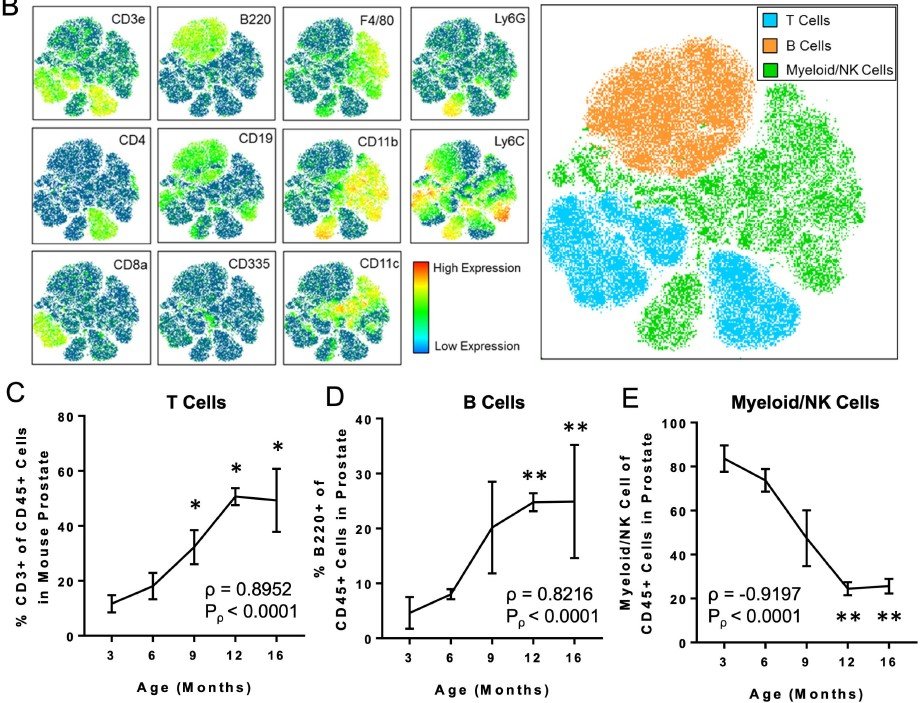
Highly multiplexed immune profiling throughout adulthood reveals kinetics of lymphocyte infiltration in the aging mouse prostate
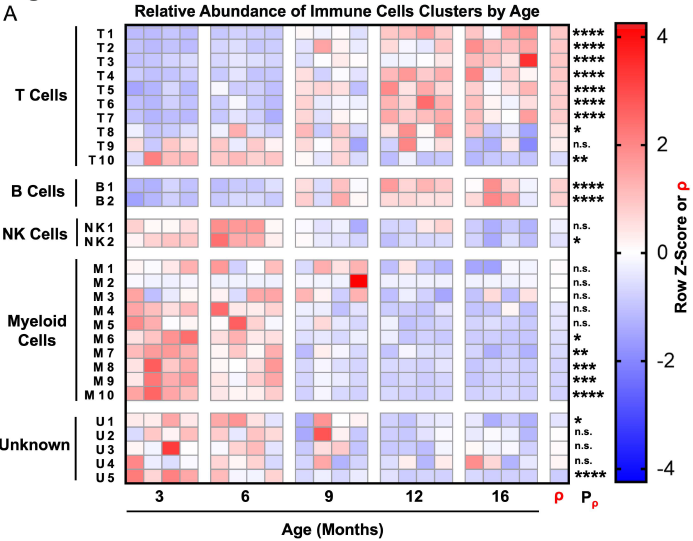
In a study published in Aging, we asked whether features of aging in the prostate emerge predominantly in old age or whether these features emerge gradually over time. Aging is a significant risk factor for disease in the prostate, and defining the kinetics of age-related changes is critical for identifying regulators of aging and evaluating interventions to slow the aging process and reduce disease risk. Using highly multiplexed immune profiling and time-course analysis, we tracked the abundance of 29 immune cell clusters in the aging mouse prostate. Early in adulthood, myeloid cells comprise the vast majority of immune cells in the prostate. Between 6 and 12 months of age, there is a profound shift towards a T and B lymphocyte-dominant mouse prostate immune microenvironment. Comparing the prostate to other urogenital tissues, we found similar features of age-related inflammation in the mouse bladder but not the kidney. In summary, our study offers new insight into the kinetics of prostatic inflammaging and the window when interventions to slow down age-related changes may be most effective.
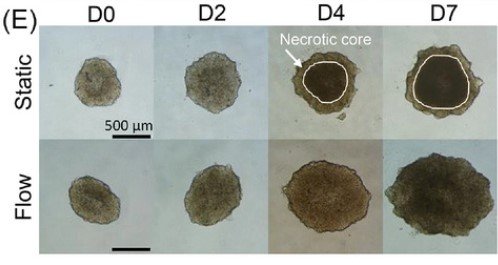
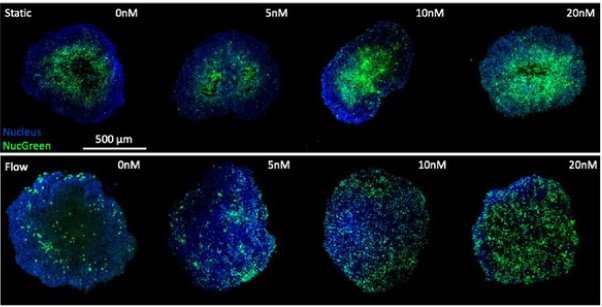
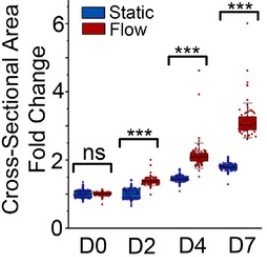
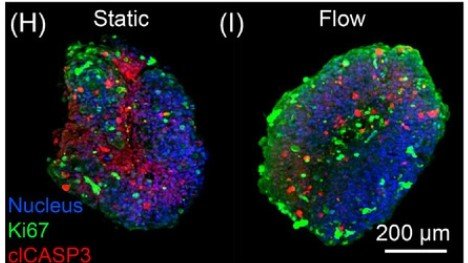
Microwell-based flow culture increases viability and restores drug response in prostate cancer spheroids
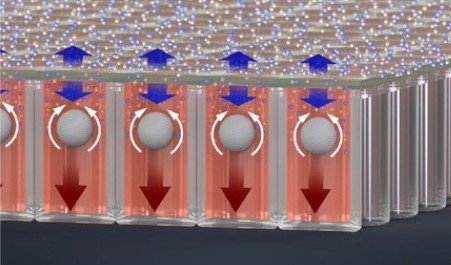
In collaboration with Dr. Neil Lin at UCLA in the Department of Bioengineering and first author Marie Payne in the Department of Mechanical & Aerospace Engineering, we set out to develop a better 3D culture system to evaluate transcriptional and proliferative responses to anti-cancer drugs using cell line-derived spheroids. Using an innovate Microwell Flow Device (MFD) that generates in-well laminar flow around 3D tissues via repetitive tissue sedimentation, we found that spheroids grow larger and remain healthier than spheroids grown in a standard ultra-low attachment culture plate. More importantly, because spheroids cultured in the flow device maintain greater viability, we can identify a greater dynamic range of response to anti-cancer drugs. This project has been a fruitful collaboration and has led to several follow-up studies to maintain viability of prostatic tissues and evaluate response to genetic and pharmacological modulation using the microwell flow device.
EXPANSION OF LUMINAL PROGENITOR CELLS IN THE AGING MOUSE AND HUMAN PROSTATE
In a study published in Cell Reports, co-first authors Preston Crowell and Jonathan Fox from the Goldstein Lab led a team of researchers at UCLA and UT Southwestern to determine the mechanisms by which aging increases disease risk in the prostate. The group determined that a specific type of progenitor cell (luminal progenitor cell) is increased in the aging mouse and human prostate. The luminal progenitor cells have previously been shown to be able to develop into prostate cancer in response to oncogenic insults, which means that there are more cells at risk for developing into cancer in the aging prostate. Future studies will be aimed at determining what causes the age-related increase in luminal progenitor cells so that we can develop strategies to slow or prevent this process and reduce the risk of disease in the prostate. Read the press release here.
Mass cytometry reveals species-specific differences and a new level of complexity for immune cells in the prostate
In a study published in the American Journal of Clinical and Experimental Urology, Jonathan Fox and colleagues at UCLA utilized mass cytometry to characterize immune cells in the mouse and human prostate. The group used mass cytometry and software analysis tools to elucidate a greater degree of immune cell heterogeneity in the mouse prostate than previously reported. The authors found fundamental differences between the immune compartment of the mouse and human prostate, as the mouse prostate is myeloid-dominant and the human prostate is lymphoid-dominant. Finally, the study revealed considerable differences in the immune compartments between patients. Future studies will be aimed at determining the functional role of various immune populations in prostate aging and tumorigenesis.